Revision of MDCG 2020-16 under Regulation (EU) 2017/746
On Feb 11, 2023, MDCG 2020-16 Guidance on Classification Rules for in vitro Diagnostic Medical Devices under Regulation (EU) 2017/746 has been updated to revision 2. The changes are as following:
1.Change to Rule 1
before change : Highly virulent pandemic influenza virus.
after change : Highly virulent influenza virus.
2.Change to Rule 3 General
before change : Rule 3 covers a range of devices as reflected in its indents (a)-(m). Devices falling under Rule 3 (when not classified as Class D according to rules 1 & 2) are to be classified in class C, irrespective of the indent applied. It may be possible for a device to fall under more than one Rule 3 indent. Where this is the case, the most appropriate indent should always be applied, based on the intended purpose of the device.
after change : Rule 3 covers a range of devices as reflected in its indents (a)-(m). Devices falling under Rule 3 (when not classified as Class D according to rules 1 & 2) are to be classified in class C, irrespective of the indent applied.It may be possible for a device to fall under more than one Rule 3 inden
3. Change to Rule 3 (f)
before change :
The identification of patients may comprise a quantitative or qualitative determination of specific markers.
Such specific markers can be present in healthy subjects and/or in patients.
The emphasis ‘before and/or during treatment’ implies that CDxs may be intended to be applied before a treatment with a corresponding medicinal product is initiated, or during treatment, to identify if (still) the patient is (a) likely to benefit from the corresponding medicinal product or (b) likely to be at increased risk of serious adverse reactions.
Devices that are intended to be used for monitoring treatment with a medicinal product in order to ensure that the concentration of relevant substances in the human body is within the therapeutic window are not considered to be CDxs
after change :
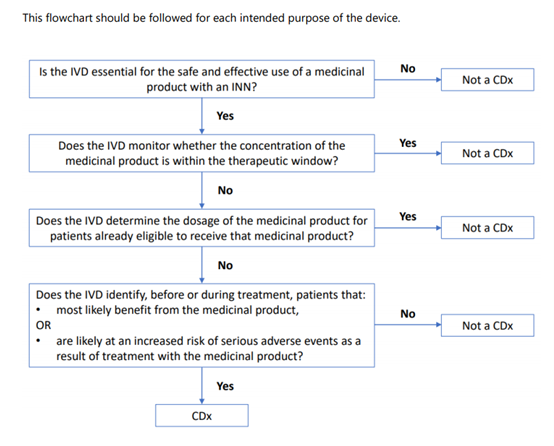
For a device to be defined as a CDx, there should be a link to a medicinal product with an International Non-proprietary Name (INN) .
The identification of patients may comprise a quantitative or qualitative determination of specific markers.
Such specific markers can be present in healthy subjects and/or in patients.
The emphasis ‘before and/or during treatment’ implies that CDxs may be intended to be applied before a treatment with a corresponding medicinal product is initiated, or during treatment, to identify if (still) the patient is(a)likely to benefit from the corresponding medicinal product or (b)likely to be at increased risk of serious adverse reactions.
Devices that are intended to be used for monitoring treatment with a medicinal product in order to ensure that the concentration of relevant substances in
the human body is within the therapeutic window are not considered to be CDxs (e.g. devices intended for blood glucose monitoring, devices intended for
measurement of cyclosporine concentration in blood, devices intended for measurement of metabolites of a medicinal product).
Devices intended to determine quantitative or qualitative specific marker(s) to establish the dosage of a particular medicinal product, for patients that are already eligible to receive that medicinal product, are not considered to be CDxs. For example, devices intended to measure creatinine concentration can be used for estimating kidney function to determine the optimal dosage of medicinal products with renal elimination. Another example is devices identifying CYP2D6 or CYP2C19 genotypes of a patient to determine the appropriate dosage of an already prescribed medication.
Annex II to this guidance provides a flowchart to help determine whether an IVD is a CDx.
4.Add Annex II to help applicant determine whether an IVD is a CDx.
Other News
-
Industry News2023/02/18
Revision of MDCG 2020-16 under Regulation (EU) 2017/746

